QMS Software for the Medical Device Industry
Customizable QMS Software Solutions for Your Needs
At QMLogic, we help MedTech companies unlock the full potential of QMS automation and digital transformation. Our electronic QMS (eQMS) software solutions combine expert consulting with platforms like Microsoft SharePoint, Power Platform, Confluence, and Jira. We also build custom eQMS software tailored to your processes, always audit-ready, validated, and compliant with MedTech regulations.
- Microsoft-Based eQMS & Power Platform
- Atlassian-Based QMS Software
- Custom and Hybrid eQMS Software
- AI-Enhanced Quality Management Systems

The best QMS is not one that makes you adapt, it’s the one that adapts to you.
We believe that Quality Management System Software should not force your organization to change the way it works. Instead, it should grow with you, adapt to your workflows, and seamlessly fit into your environment.
We offer:
- Customizable QMS Software Solutions designed around your processes
- Secure QMS architecture ensuring data integrity and compliance
- Adaptable QMS Software that evolves as your organization evolves
Smarter QMS Software
QMS Solution Beyond Ready-Made Packages
Most off-the-shelf QMS Software tries to cover everything with a rigid feature view. This often creates inefficiencies, unnecessary complexity, or the need to change how people in your organization work.
At QMLogic, our approach is different.
- We integrate with your existing tools instead of replacing them, and build additional functionalities just where it is needed
- We connect departments and systems into one functioning ecosystem
- We reduce cost and complexity while improving clarity, communication, and efficiency
QMS Software Solutions Tailored to Your Technology Environment
We build QMS Software solutions that adapt to your organization, not the other way around.
Our strength lies in flexibility: whether your company operates on Atlassian, Microsoft, or other software solutions in a hybrid environment, we design a Customizable QMS Environment that integrates seamlessly with your business.


What You Gain with QMLogic QMS Digitalization
Optimized Efficiency
Automate repetitive quality tasks to save time and reduce manual effort.
Fewer Errors, More Accuracy
Minimize risk with automated workflows and consistent, validated data.
Simplified Audits & Full Traceability
Real-time dashboards and audit trails for effortless compliance.
Reduced Costs & Complexity
Optimize workflows, minimize redundancy, and improve team productivity.
Centralized Insights
Bring all your quality data together for better visibility and faster decision-making.
Our Core eQMS Technology Foundations & Software Tools
We build compliant, scalable and connected Quality Management Systems (eQMS), adapted to your company’s size, needs, and existing setup. Instead of forcing a single technology, we build on what you already use or introduce new tools where they bring real value.
Our implementations are designed around compliance with core standards and regulations, such as ISO 13485 or 21 CFR 820, and beyond. We ensure your QMS is digitalized, audit-ready, and future-proof. By leveraging proven platforms and our regulatory expertise, we deliver compliant eQMS solutions faster, with minimal disruption to your ongoing operations.
Your tools, your processes, our expertise, transformed into a digital QMS.
QMS Built on Your Infrastructure
We design QMS environments built on Microsoft technologies, using SharePoint, Power Platform (Power Apps, Power Automate, and Power BI). We deliver a seamless, secure, and compliant QMS automation system, designed around your exact needs.
Full Ownership & No Vendor Lock-In
No licensing traps, hidden dependencies, or external vendor lock-ins, the system is fully yours to manage, adapt, and evolve.
Data Stays Inside Your Company
We don't store or access any data once the system is under your control. You can leave anytime, without losing control of your records and manage your own administration.
MedTech Compliance Built-In
We design with ISO 13485, FDA 21 CFR Part 820, Part 11, and MDR in mind, ensuring every workflow and form meets regulatory requirements from the start.
Microsoft 365 Integration
Our solutions are natively built on PowerApps, SharePoint, and Power Automate, integrating seamlessly with your existing Microsoft environment for a unified QMS ecosystem.
Modular & Scalable
Start small with essential workflows and extend functionality step by step. No need to build everything at once, your QMS can grow with you.
Custom QMS Solutions
Beyond Atlassian and Microsoft environments, we create custom QMS software and integrations tailored to your exact needs. This can mean:
- Connecting Atlassian and Microsoft worlds where necessary
- Building new software components that fill functionality gaps
- Developing standalone QMS modules for specific business needs
- Extending interoperability with tools such as SAP, Polarion, IBM DOORS
When standard tools can’t meet your needs, we design custom eQMS software from the ground up. Our solutions are audit-ready by design, aligned with ISO 13485, FDA 21 CFR, and EU MDR, and tailored to your product lifecycle, regulatory framework, and internal SOPs.
Enhancing Microsoft Solutions

We extend SharePoint and Power Platform with advanced capabilities using:
- SPFx, Graph API & Custom Connectors for deeper functionality
- New apps, dashboards, and integrations built on Microsoft 365
- Expanded automation and reporting without vendor lock-in
Fully Custom Software Development
For organizations needing complete flexibility, we build standalone or cloud-based solutions:
- Technologies: React, Java, .NET, Next.js, Azure and more
- Full-stack development for any process automation
- Automate any QMS or business process without platform limits
- Designed for ISO 13485, FDA 21 CFR, and EU MDR compliance from the start

AI-Based QMS Features
AI is the intelligence layer that connects platforms, processes, and people.
At QMLogic, we embed AI into QMS environments, transforming simple automation into intelligence. Our AI-driven solutions extend beyond simple workflows, bringing smart assistance, predictive analytics, and self-optimizing processes to your quality ecosystem.
What AI Bring to Your QMS
- Automated QMS & TechDoc content creation for faster, consistent documentation
- Document consistency & template compliance with instant AI-based checks
- Predictive project tracking & bottleneck detection across your quality processes
- AI-powered knowledge assistants for quick access to product and process data
AI enables your QMS to think ahead — detecting risks, identifying trends, and ensuring traceable, audit-ready documentation.
The result: fewer errors, stronger compliance, and faster, smarter decisions.
At QMLogic, we do not offer just predefined or limited AI features. In the same way we design customizable QMS Software Solutions, we also create custom AI-augmented functionalities for specific needs of each organization.

Practical AI that strengthens compliance and accelerates performance.
AI for Multiple Quality Management System Areas
Control of documents and records
Automatically checking consistency between procedures, templates, and records
Design control processes
Supporting requirement engineering, design specification, validation, and verification activities
Product lifecycle and risk management
Identifying gaps, trends, and risk correlations
Cybersecurity processes
Improving incident monitoring and risk prioritization
Audits, data analysis, and management reviews
Assisting with data preparation, audit traceability, and evidence collection
AI transforms QMS from automation to intelligence, with predictive analytics, risk detection, and smart documentation support. The result: fewer errors, stronger compliance, and faster decisions.
QMS Software Tools
Real-World Use Cases of Our Custom QMS Solution
We don’t just talk about automation, we deliver it. Our solutions are born from real-world challenges we’ve solved for MedTech companies, replacing slow, manual processes with fast, compliant, and fully digital systems.
Project Roadmap – Unified Documentation & Project Management
A custom-built solution that streamlines documentation and project workflows across departments.
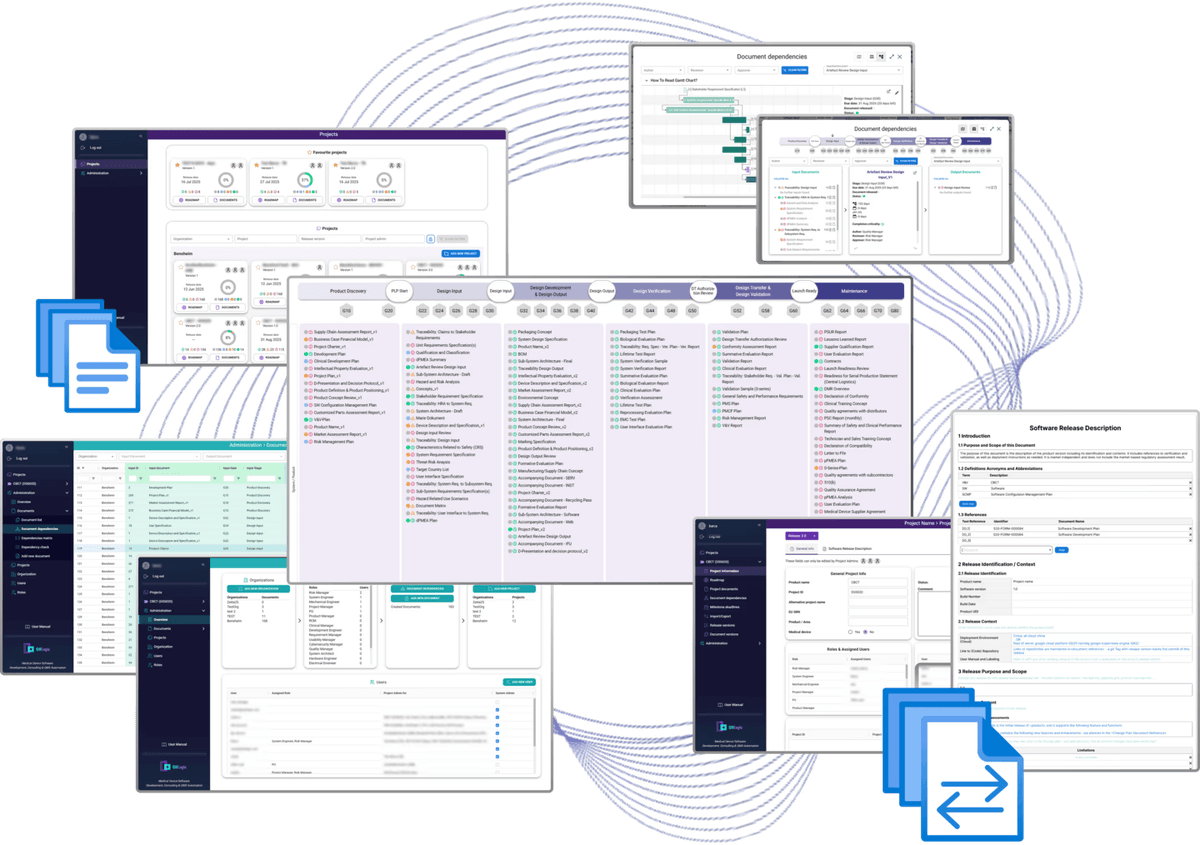
- Centralized control over documents, versions, and change management

- Manage project releases and collaboration across multiple teams

- Track document progress and criticality for project managers

- Built-in roles and permissions for secure workflows

- Confluence integration for easy export and consistency checks across document versions

Computer System Validation App – Automation at Speed
A low-code app designed to fully digitalize and automate software tool validation.
- SharePoint as a secure storage backbone

- Power Apps UI for simple, guided navigation

- Power Automate for alerts, notifications, and automated document exports

- PDF export for validation records

- Built-in statistics and dashboards to track validation progress in real time


Application of AI in QMS
AI in QMS isn't just about automation, it's about intelligence, foresight, and compliance at scale. By embedding AI into our solutions, we enable MedTech companies to move from manual oversight to predictive, self-checking systems.
Smarter Decisions, Backed by Real-Time Insights
Our interactive, AI-augmented dashboards provide valuable insights across your organization, enabling you to make the right decisions quickly and confidently, without tedious manual data collection.
With just a few clicks, you gain access to:
- Real-time analytical overviews
- Automated performance tracking
- Intelligent summaries that support management reviews and continuous improvement

Empower your quality experts with AI, make your QMS smarter and responsive!
Examples of AI-Driven Use Cases in QMS
QMS & Product Knowledge Chatbots
Provide instant answers and guidance to your teams, directly from your validated QMS data.
Automated Document Generation
Speed up compliance reporting, audits, and technical documentation with AI-assisted drafting.
Audits & Management Reviews
Generate real-time summaries, findings, and corrective action insights.
Requirements Alignment & Quality Assurance
Cross-check design controls, CAPAs, and requirements for consistency and compliance.
Predictive Project Tracking & Bottleneck Detection
Spot risks before they turn into delays or non-compliance issues.
Document Consistency & Template Compliance
Ensure every record meets QMS and regulatory standards automatically.
With AI integrated into your QMS, quality management becomes proactive — a system that learns, predicts, and supports compliance every day.
QMS Software Compliant with ISO 13485 and 21 CFR 820
Our team have guided organizations through multiple certifications and audits, ensuring solutions meet global standards. Compliance always remains in focus. That’s why our solutions are designed to meet regulatory requirements from the ground up.

ISO 13485
for medical device quality management

ISO 80002-2
Computer System Validation

ISO 27001
for data protection and information security

ISO 42001
Artificial intelligence – Management system

21 CFR 820 (QMSR)
for FDA-regulated environments

Extending Compliance Beyond the Core QMS
In medical device development and manufacturing, compliance extends beyond ISO 13485 and 21 CFR 820. That’s why our eQMS Solutions also cover broader regulatory and lifecycle requirements essential for maintaining compliance throughout the entire medical device life cycle.

21 CFR 820 (QMSR)
for FDA-regulated environments
- Medical Device Reporting (MDR) – 21 CFR 803
- Medical Device Tracking Requirements – 21 CFR 821
- Postmarket Surveillance – 21 CFR 822

EU MDR
Medical Device Regulation 2017/745
- Post-Market Surveillance
- Vigilance System
- Post-Market Clinical Follow-Up (PMCF)
- Periodic Safety Update Reports (PSUR)
Built-in compliance by design. Aligning innovation with assurance, seamlessly
These functions ensure that your QMS not only manages internal processes but also supports regulatory submissions, incident reporting, field actions, and market surveillance activities — all within a unified, secure environment.

Your Right Partner in QMS Software Implementation
Building eQMS that Works for You and Auditors
At QMLogic, we don’t just talk about QMS digitalization; we have delivered it successfully across industries and technologies. We have designed and implemented Quality Management Systems from the ground up several times.

Experience Across Platforms and Technologies
We have created digital Quality Management Systems on different technological backbones:
- Atlassian Suite (Confluence & Jira) for agile QMS workflows

- Microsoft tools (SharePoint, Power Apps, Power Automate, Power BI) for scalable, enterprise-wide QMS solutions

- Custom-built software tailored for QMS documentation, design controls, risk management, or complete business operations

Beyond standalone systems, we have enabled interoperability with tools such as:
- SAP ERP systems

- Requirements engineering tools like Polarion

- Document management platforms like SmartSolve

Compliance You Can Trust
Our solutions have been audited multiple times by notified bodies and external auditors and confirmed to be:
- Fully compliant with ISO 13485, 21 CFR 820, IEC 62304, and ISO 14971
- Designed to ensure compliance with cybersecurity and data privacy requirements defined by ISO 27001 and IEC 81001-5-1
- Ready for regulatory scrutiny, certification audits, and global compliance needs
We go beyond audit readiness, delivering systems that align compliance with business effectiveness, cost efficiency, and sustainable growth.
Testimonials | Expertise in QMS Digitalization
Vaclav did support our journey to become ISO 13485 certified with relentless work put into great processes, great communication and detailed and to the point conversations with the teams, individual members - but also our external auditors. He was of great support to move our QMS to the next level. He was the key person behind the move away from paper / scan based documents towards a digital setup.If you are in need for a structured person with great communication skills, a good understanding of the regulatory environment, I can only recommend to reach out to Vaclav. Lucky you if he's not booked ;-) Besides all the success he's an enjoyable and humble character. Great experience to having worked with him.
Vaclav and I worked together on risk management activities of medical device development projects. His ability to quickly grasp the challenges and to accomplish a task/project is remarkable. Additionally, his ability to propose and develop software solutions to improve projects’ efficiency is commendable. I find Vaclav to be flexible, dependable, and quality centric. Hence, I really enjoyed working with Vaclav and looking forward to associate with him again.
Our Vision of a Modern eQMS for Operational Excellence
We at QMLogic design QMS Software with one principle in mind: no two organizations are the same. Every company has its own way of working, its own companion software. It operates within various regulatory frameworks and medical device areas, including mechanical, electronic, hardware, software, and cloud-based software. And every company treats design and development, suppliers, customers, and many more differently.
That’s why there cannot be a single ready-made QMS that fits everyone. In reality, so-called “catch-all” solutions are closer to science fiction or brave marketing slogans than practical reality.
Instead, we focus on Customizable QMS Software Solutions that adapt to your organization. We build systems that are interoperable with your existing tools, reflect your workflows, and grow as your company evolves.
With this philosophy, let’s look at the key areas of the Quality Management System, as described in ISO 13485 and 21 CFR 820, and how we envision them in a modern QMS Software environment.
Your QMS should not be where documents go to die. It should be where processes come alive.
QMS Software as a Living Environment
Many organizations start with control of documents and records as the foundation of their Quality Management System. While this is an essential cornerstone, a QMS cannot be reduced to simple document storage. Without automation, integration, and real-time accessibility, a QMS becomes nothing more than an archival tool, failing to add real value to the organization.
At QMLogic, we build Customizable QMS Software Solutions that reflect real workflows, automate daily tasks, and create evidence of functioning processes. We believe in systems that live, evolve, and drive efficiency, while staying compliant with applicable standards and regulations.
Every effective QMS begins with document and record control. This is the starting point for compliance audits and one of the most critical areas of regulatory scrutiny.
But in our view, document control is more than archiving. The eQMS must provide:
- An effective way for reviewing and approving documents throughout the organization
- Automated notifications about updates to processes, templates, and work instructions
- Collaborative content creation, not just static storage
Questions & Answers
Answer: Yes, we can help you choose the right solution for you, either because you just decided to leave your current vendor, or you are first establishing a quality management system.
Together, we will analyze your current situation and define the requirements that are most important to you, and we can advise you which quality management system solution to choose.
We have the most experience in building quality management systems around the Atlassian or Microsoft suite, but we can evaluate any other software solution that would fit your needs.
For specific requirements and organizational setups, we also provide custom-made software solutions, fully developed by us.

